The Importance of pH and Liming Material
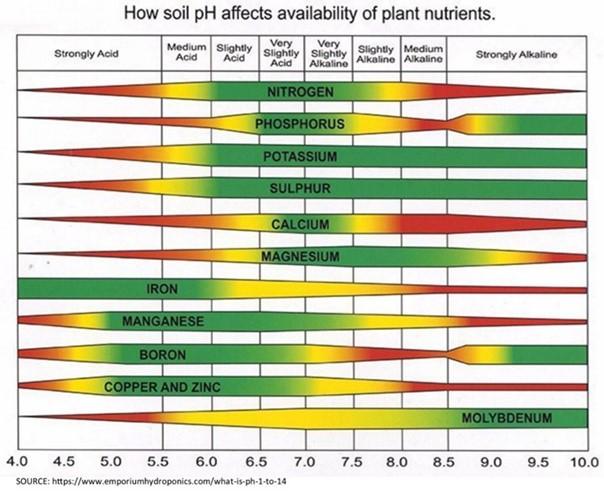
If I were stranded on a desert island and could do only one part of the soil test to determine how to grow food, I would test for pH.” This statement, made by Dr. Doug Beegle, my soil fertility professor, highlighted how important soil pH is. For most agronomic crops, the ideal pH is between 6.0 and 6.5. Alfalfa and barley prefer a bit higher pH of 6.5-7.0. Between the pH of 6 and 7, nutrient availability is at its optimum. Outside that range, key nutrients such as nitrogen, phosphorus, and potassium become more tightly bound to other nutrients and unavailable for the crops to take up (Figure 1). Below 6.0, nutrients such as iron, copper, and aluminum become more available, and in some cases could result in toxicity to the crop.
Over time, the pH of soil naturally decreases. So, to increase the pH, we add lime. The soil test results will provide the amount of lime needed to increase pH to the optimum level. The lab uses the current soil pH and acidity of the soil to determine how much lime is needed. (Your soil test may report the acidity, which is measured in milliequivalents per 100 grams [meg/100 g]).
Let’s say your soil test result says that you need 2 tons of lime per acre to increase the pH to 7.0. Does that mean you can put on 2 tons of whatever liming material you like best? Not quite. The results are given based on the assumption of using calcium carbonate, which is considered pure limestone and given a rating of 100% calcium carbonate equivalent, or CCE. All other liming materials are compared to calcium carbonate and given their own CCE (Table 1). For example, burned lime has a CCE of 178. This means that it has more acid-neutralizing activity than pure calcium carbonate; therefore, less material can be used to obtain the same neutralizing activity as pure lime. Wood ashes, on the other hand, has a CCE of 40; therefore, more material needs to be applied in order to adjust the pH.
| Liming material | Calcium carbonate equivalent (CCE) | Equivalent to one ton pure limestone |
|---|---|---|
| % | pounds | |
| Ground limestone, calcitic limestone, calcitic lime, calcite, hi-cal limestone, calcium carbonate | 100 | 2,000 |
| Burned lime, quick lime, unslaked lime, calcium oxide | 178 | 1,120 |
| Hydrated lime, builders' lime, slaked lime, calcium hydroxide | 134 | 1,490 |
| Dolomitic limestone, hi-mag limestone, calcium magnesium carbonate | 95-109 | 1,830-2,100 |
| Ground shells | 80-90 | 2,200-2,500 |
| calcium silicate slag | 70-80 | 2,500-2860 |
| Blast furnace slag, basic slag | 67-75 | 2,670-2,990 |
| Flue dust | 96 | 2,080 |
| Marl | 40-90 | 2,220-5,000 |
| Wood ashes | 40 | 5,000 |
Don’t forget to take the price into consideration when comparing liming materials! For example, if ground shells are really cheap, that’s great; but it has a lower CCE, so you’ll need to apply more.
For more information, click here (Soil Fertility Management PDF) to read the Soil pH Management and Determining Lime Rates fact sheet.
This article appears on April 2022, Volume 13, Issue 1 of the Agronomy news