
FS-1193 | February 2022
Understanding Glyphosate and Other Pesticides
Glyphosate is a uniquely effective herbicide, used for over 40 years to control more than 230 weeds. Recent concerns about the widespread use of glyphosate and its impacts on human health and the environment have propelled this herbicide into the national spotlight. This article provides an overview of glyphosate, and the regulations in place to ensure there are no unreasonable risks to human health when used according to label directions.
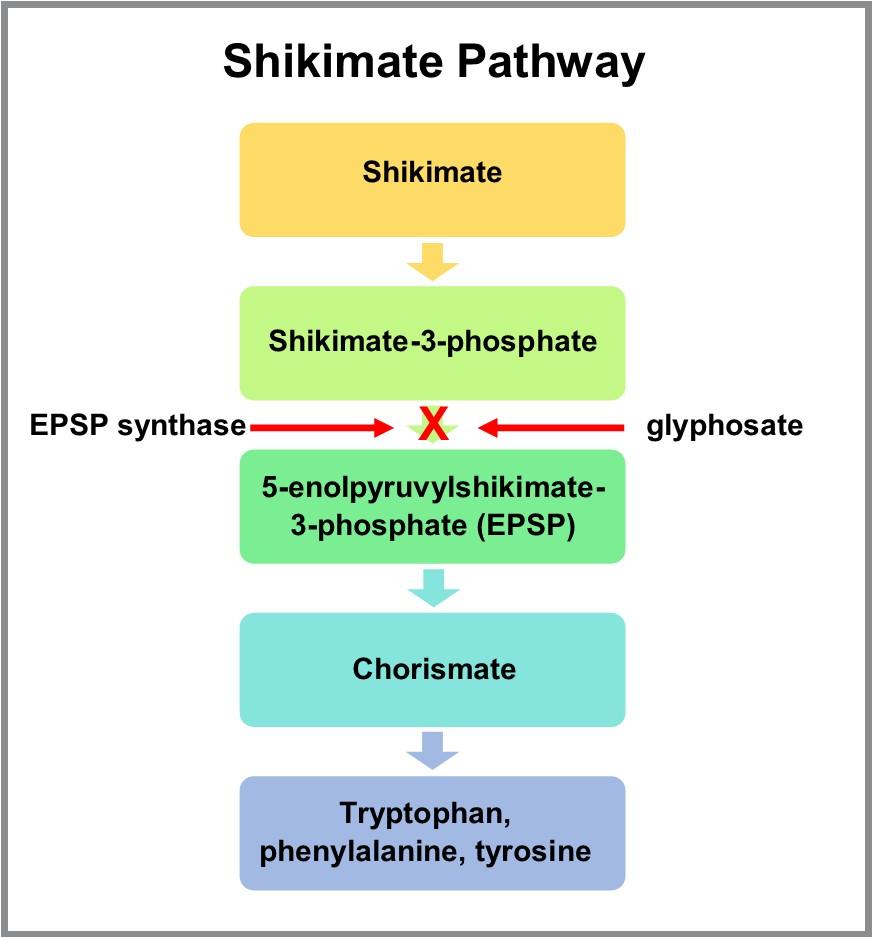
Glyphosate inhibits EPSP synthase, an enzyme in the shikimic acid pathway (Figure 1). Inhibiting this enzyme prevents plants from producing the amino acids phenylalanine, tryptophan, and tyrosine, which are required to synthesize proteins and lignin in plant cells (Shaner 2014). Without these amino acids, plants lack a support structure for growth and development, and eventually die. Because glyphosate is non-selective, most plants sprayed are impacted. Humans and other animals do not have the shikimate pathway and cannot synthesize phenylalanine, tryptophan, and tyrosine. Furthermore, the glyphosate molecule does not contain functional groups that would interact with biomolecules other than EPSP synthase (Berry 2020).
Pesticides are Regulated
Glyphosate, like any other pesticide, is intended to prevent, destroy, repel or mitigate a pest. Pesticide is a broad term which includes not only herbicides, such as glyphosate, but also fungicides, insecticides, and even everyday cleaning items such as disinfecting wipes. Regulations such as the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA); Federal Food, Drug and Cosmetic Act; and Food Quality Protection Act help to ensure proper pesticide use.
All pesticides sold or distributed within the United States must be registered with the Environmental Protection Agency (EPA) and each state where they are sold, and have specific labeling requirements for their intended use. Failure to use a pesticide properly can result in civil and criminal penalties. The Food and Drug Administration, the U.S. Department of Agriculture, and the Occupational Safety and Health Administration are also involved in upholding food safety and protecting agricultural workers. FIFRA requires that a pesticide not cause “unreasonable adverse effects on the environment.” These effects are defined as “unreasonable risk to man or the environment, taking into account the economic, social and environmental costs and benefits of the use of any pesticide.”
Both the active ingredient of a pesticide (such as glyphosate) and any commercial formulations of that active ingredient (such as Roundup®) must go through a registration process involving over 243 base studies (National Archives 2021). These studies include product chemistry and performance, data from studies to determine hazard to humans and domestic animals, data from studies which determine hazard to non-target organisms, post application exposure studies, applicator/user exposure studies, pesticide spray-drift evaluation, and environmental fate. From these studies, the EPA conducts risk assessments associated with using a pesticide as intended, based on both the inherent toxicity (hazard) of a pesticide and how humans or animals may be exposed.
Toxicity of Pesticides Can Be Acute or Chronic
Acute toxicity describes the adverse effects of a substance resulting from either a single exposure or multiple exposures in a short time period. Acute toxicity studies establish toxicity levels of pesticide to test organisms through ingestion, inhalation, and dermal exposure. This information is then compared with measured or estimated amounts of pesticide residues in the environment to assess the potential effects on non-target organisms. The lethal dose (LD₅₀) in mg of pesticide per kg of body weight or lethal concentration (LC₅₀) in mg pesticide per L of air required to kill half a test population is the standard metric for evaluating acute toxicity. Substances with lower LD₅₀ values are more toxic because less is needed to cause harm, but the amount and manner in which that substance is consumed will determine the overall risk of harm. For example, the LD50 of pure glyphosate is lower than that of pure caffeine (Table 1), but drinking a formulated glyphosate-product, such as Roundup®, carries greater risk than drinking a cup of coffee. It is unlikely, however, that a person would intentionally drink enough Roundup® or consume enough pure caffeine to cause harm.
| Substance | LD₅₀ (mg/kg) |
|---|---|
| Nicotine | 9 |
| Caffeine | 192 |
| Tylenol | 338 |
| Table salt | 3,000 |
| Glyphosate | 5,600 |
Chronic toxicity describes the adverse effects related to delayed exposures of a chemical over time (EPA 1993). Chronic toxicity studies establish the acceptable daily intake (ADI) or Reference dose (RfD), estimating how much pesticide a person can be exposed to over a lifetime at which there is no increase in the frequency or severity of adverse health effects. Animal feeding studies are used to calculate the highest level at which there is no increase in the frequency and severity of adverse health effects over a lifetime (No Observed Adverse Effect Level, NOAEL). From these studies, the EPA models how much of a pesticide the average person will be exposed to, and builds in serious safety or uncertainty factors before establishing an ADI. At least two, 10-fold additional safety factors, are required to establish an ADI, one to account for differences in human and animal physiology, and one to account for people who may be at higher risk from exposure to a pesticide. Additional modifying factors can also be included based on other scientific uncertainties.

The ADI of a substance is then used to establish tolerances, also known as maximum residue limits (MRLs), the maximum amount of pesticide residue safely allowed in or on human food or animal feed. Tolerances are not directly related to safety, but work as indicators of good agricultural practices. The presence of a detectible pesticide residue does not mean the residue is at an unsafe level. Tolerances are only established if current and proposed uses of a pesticide represent a reasonable certainty of no harm (CAST 2019). Tolerances are often set from 100 to 1000 times lower than the ADI, ensuring consumer safety and that only the necessary amount of a particular pesticide is applied to achieve the desired effect. In setting tolerances, it is assumed the maximum allowable rate of a pesticide will be used and the maximum number of allowable applications will be made at the shortest allowable interval before harvest. Tolerances are not only set for a particular pesticide, but also for any byproducts, metabolites, and reaction products resulting from a pesticide being broken down after application.
When setting tolerances, the EPA considers 1) the pesticide’s toxicity and all its breakdown products; 2) how much of the pesticide is applied and how often; 3) how much of the pesticide remains on the final consumer product; and 4) all possible routes of exposure, such as crop use, drinking water, and residential exposure, for example (EPA 2021a).
Dose and Exposure Are Critical Factors in Considering Pesticide Safety
“The dose makes the poison” -Paracelsus
Although glyphosate use has increased over the past 25 years, constant exposure to glyphosate at high enough levels is needed to produce a negative effect. Glyphosate exposure can occur via direct exposure on the skin, inhalation, or through ingestion (Henderson et al. 2010a). Glyphosate does not easily pass through human skin (Wester et al. 1991), however, and is not volatile or likely to become a vapor after it has been sprayed (Shaner 2014). Glyphosate absorbed by the skin or ingested is excreted relatively quickly without being changed into another chemical (Henderson et al. 2010b; Williams et al 2000; WHO 1994).
According to the EPA, the acceptable daily intake for glyphosate is 1.0 mg/kg/day (EPA 2015). Average exposure to glyphosate, including through dietary intake, however, is less than 0.0045 mg/kg/day (Niehmann et al. 2015; Stephenson and Harris 2016; Whitford et al. 2007), or 222 times lower than the amount expected to cause chronic illness and over 1 million times lower than the amount expected to cause acute illness. Additionally, a 2017 report in corn and soybean, crops genetically engineered to tolerate glyphosate, found that no samples had glyphosate residues above EPA tolerance levels, and 82% of corn and 60% of soybean samples did not have any detectable residues (FDA 2017).
These factors can also help to understand an individual’s risk for developing cancer. The International Agency for Research on Cancer (IARC), a sub-branch of the World Health Organization (WHO), has classified glyphosate as a “Category 2A probable human carcinogen” (IARC 2015). At least 15 regulatory and research agencies have concluded, however, that glyphosate does not pose a cancer risk, when used as labeled. These agencies include the EPA (2017), European Food Safety Authority (2015), and Health Canada (2017), in addition to 2 reports from the World Health Organization (International Programme on Chemical Safety, 1994; Guidelines for Drinking-water Quality, 2004), and a joint meeting of the WHO and United Nations Food and Agriculture Organization (2016). The difference in IARC findings versus others is due to how glyphosate was evaluated. Regulatory agencies conduct risk assessments, the likelihood that a substance will cause harm based on dose and exposure. IARC conducted a hazard assessment, the potential to cause harm, regardless of dose or exposure. IARC considers eating red meat and drinking hot beverages to be just as hazardous as glyphosate exposure, and it considers drinking alcoholic beverages, exposure to sunlight, and tobacco use to be more hazardous than glyphosate (Figure 3).

Understanding the difference between hazard and risk is important as all chemicals and everyday activities have potential risks. The level of exposure to a particular substance increases the risk of harm, regardless of how benign the substance may seem. For example, water is consumed every day, but consuming too much water can result in over-hydration and water poisoning. Even IARC has stated the probability of developing cancer from glyphosate exposure will depend on factors such as type and extent of exposure and the strength of the effect of the agent (IARC 2016). IARC did not, however, state the amount and extent of glyphosate exposure needed to cause cancer.
Once a pesticide completes the registration process, it must be re-registered every 15 years to ensure it meets current scientific and regulatory standards. In addition, any pesticide can be reevaluated outside of this 15-year cycle if there is new data requiring review. The EPA completed its interim re-registration decision of glyphosate in 2020 and found that when used correctly:
- There are no risks to human health from current uses of glyphosate.
- There is no indication that children are more sensitive to glyphosate.
- There is no evidence that glyphosate causes cancer in humans.
- There is no indication that glyphosate is an endocrine disrupter.
- Trace amounts of glyphosate on food are not of concern for the consumer (EPA 2021b).

Glyphosate remains an important weed management tool but its continued use raises important safety questions. Exposure to glyphosate, however, can have the same or less risk as many of the foods consumed, activities participated in, and processes a person is exposed to every day. Furthermore, regulations put in place for glyphosate as well as other pesticides ensure that the amount of a pesticide a person is exposed to is significantly lower than the amount which would cause harm (Figure 4).
References
Berry, C. (2020). Glyphosate and cancer: the importance of the whole picture. Pest Mgmt Sci 76:2874-2877.
Council for Agricultural Science and Technology (2019). Interpreting Pesticide Residues in Food. Issue Paper 66. CAST, Ames, Iowa.
U.S. Environmental Protection Agency. (2021a). Setting Tolerances for Pesticide Residues in Foods. https://www.epa.gov/pesticide-tolerances/setting-tolerances-pesticide-residues-foods. Accessed 29 September 2021.
U.S. Environmental Protection Agency. (2021b). Glyphosate. https://www.epa.gov/ingredients-used-pesticide-products/glyphosate. Accessed 12 December 2021.
U.S. Environmental Protection Agency. (2017). Glyphosate. Draft Human Health Risk Assessment in Support of Registration Review.
U.S. Environmental Protection Agency. (1993). Reference Dose (RfD): Description and Use in Health Risk Assessments. https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments. Accessed 29 September 2021.
European Food Safety Authority. (2015). Glyphosate: EFSA Updates Toxicological Profile. https://www.efsa.europa.eu/en/press/news/151112. Accessed 4 October 2021.
World Health Organization/Food and Agricultural Organization of the United Nations. (2016). Joint FAO/WHO meeting on pesticide residues summary report. https://www.who.int/foodsafety/jmprsummary2016.pdf. Accessed 29 September 2021.
U.S. Food and Drug Administration. (2017). Pesticide Residue Monitoring Program Fiscal Year 2017 Pesticide Report. https://www.fda.gov/media/130291/download. Accessed 12 December 2021.
Health Canada. (2017). Re-evaluation Decision RVD2017-02, Glyphosate. https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2017/glyphosate-rvd-2017-01.html. Accessed 4 October 2021.
Henderson, A.M., Gervais, J.A., Luukinen, B., Buhl, K., Stone, D., Cross, A., Jenkins, J. (2010a). Glyphosate General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/glyphogen.html. Accessed 29 September 2021.
Henderson, A.M., Gervais, J.A., Luukinen, B., Buhl, K., Stone, D., Strid, A., Cross, A., Jenkins, J. (2010b) Glyphosate Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/archive/glyphotech.html. Accessed 29 September 2021.
International Agency on Cancer Research. (2015). Some organophosphate insecticides and herbicides. IARC monographs on the evaluation of carcinogenic risks to humans. Vol 112. https://publications.iarc.fr/549. Accessed 4 October 2021.
International Agency on Cancer Research. (2016). Q&A on Glyphosate. https://www.iarc.who.int/wp-content/uploads/2018/11/QA_Glyphosate.pdf. Accessed 4 October 2021.
National Archives. (2021). Code of Federal Regulations Part 158- Data requirements for pesticides. eCFR :: 40 CFR Part 158 -- Data Requirements for Pesticides. Accessed 1 October 2021.
Shaner, D.L., Ed. (2014). Herbicide Handbook: Tenth Edition. Weed Science Society of America, Lawrence, KS.
Wester, R.C., Melendres, J., Sarason, R., McMaster, J., Maibach, H.I. (1991). Glyphosate skin binding absorption, residual tissue distribution, and skin decontamination. Fund. Appl. Toxicol. 16:725-732.
Williams, G.M., Kroes, R, Munro. I.C. (2000). Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 31:117-165.
World Health Organization. (2005). Glyphosate and AMPA in drinking-water. https://www.who.int/water_sanitation_health/dwq/chemicals/glyphosateampa290605.pdf. Accessed 15 December 2021.
World Health Organization. (1994). Environmental Health Criteria 159, Toxicological Evaluations - Glyphosate. International Programme on Chemical Safety: Geneva, Switzerland.
Whitford, F., Storm, J., Mysz, A., Alexander, B., Acquavella, J., Buhler, W., Janssen, C., Neltner, T., Burns, C., Schmidt, D., Mandel, J., Blessing, A. (2007). Farm Family Exposure to Pesticides: A Discussion with Farm Families. Purdue Extension PPP-72.
| KURT VOLLMER kvollmer@umd.edu This publication, Understanding Glyphosate and Other Pesticides (FS-1193) is a part of a collection produced by the University of Maryland Extension within the College of Agriculture and Natural Resources. The information presented has met UME peer-review standards, including internal and external technical review. For help accessing this or any UME publication contact: itaccessibility@umd.edu For more information on this and other topics, visit the University of Maryland Extension website at extension.umd.edu University programs, activities, and facilities are available to all without regard to race, color, sex, gender identity or expression, sexual orientation, marital status, age, national origin, political affiliation, physical or mental disability, religion, protected veteran status, genetic information, personal appearance, or any other legally protected class. |